lb spent in the autoclave overnight|how long does lb last after autoclave : fabrication I've prepared a fair amount of solid plant TC media myself and I don't see a huge issue with letting the mixture sit in a fridge overnight before you autoclave. Montare un autoclave può sembrare una sfida complessa, ma con la giusta .
{plog:ftitle_list}
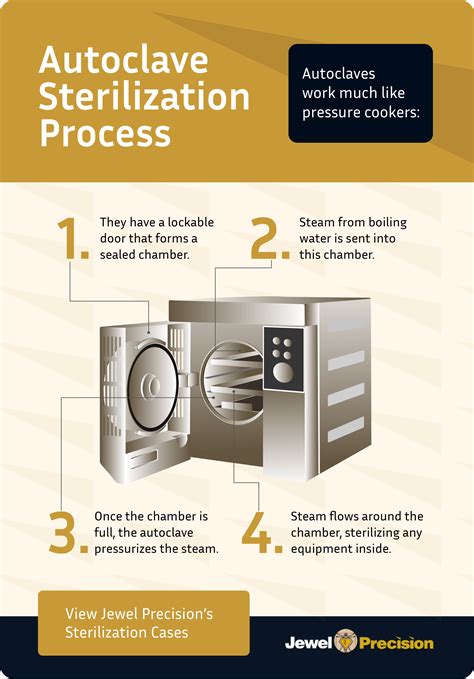
The basic principle of steam sterilization, as accomplished in an autoclave, is to expose each .60 Yd. Roll of Autoclave Tape – Indicates Sterilization for Dental Tools, Surgical Instruments & .
how long does lb last after autoclave
pompe centrifuge
how long can you store autoclaved reagent
Leaving media or buffers overnight is not a problem because after the 15 minutes it will slowly cool down to ambient temperatures and if the autoclave is still closed the entire inside will stay sterile.I've prepared a fair amount of solid plant TC media myself and I don't see a huge issue with letting the mixture sit in a fridge overnight before you autoclave.LB can be used months after autoclaving, but antibiotics break down chemically in weeks (ampicillin 1000x stocks lose their potency in a month). Even in the refrigerator Agar plates .
Use this protocol to prepare LB agar plates with antibiotic in your lab. . The autoclave tape will darken during the autoclave process if your sample has spent at least 10 min at 121 ℃. Use lab tape to label the bottle with your initials, the date, and the bottle contents. . After overnight drying, we place the plates in a plastic bag . Schematic of in vitro evaluation of bacteriophage treatment of biofilms. A bacterial inoculum is prepared from a fresh agar plate of the desired strain and incubated at proper conditions (a).The microplate is inoculated with overnight grown bacteria diluted with the desired culture medium (b).The spent culture medium is replaced with fresh medium and the desired .Not long. If you want to make large batches, I would recommend weighing out your media in advance and storing them airtight in the glassware you want them in.
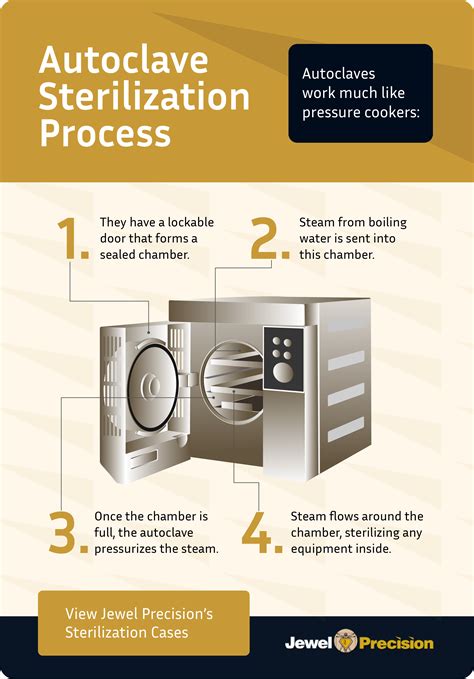
Autoclave, LB media, Agar plates Module Hours: 3.0 Effective Date: 02/01/2022 PRQs: Buffers and Stock Solutions Revision 2.0 . darken during the autoclave process if your sample has spent at least 10 min at 121 ℃. Use lab tape to label the bottle with .Luria-Bertani (LB) broth and 2X YT. Overnight Express System 1 medium Aseptically combine the following reagents Per liter: 20 ml OnEx™ Solution 1 50 ml OnEx Solution 2 . deionized water and autoclave • Adjust volume to 1 L with deionized water and autoclave TB383 Rev. C 0903 3 United States & Canada 800-207-0144
golden supplier end suction centrifugal pump
Biohazardous materials should not be left in an autoclave overnight in anticipation of autoclaving the next day. For the autoclave process to be effective in achieving sterilization, sufficient temperature, time and direct steam contact are essential. . Usually 121 degrees Celsius at 15 pounds-force per square inch (lbf/in 2 or psi) for a . Transfer colony into 25 mL of LB broth in 250 mL flask. Incubate culture of 6-8 hours at 37°C with vigorous shaking (250-300 rpm). Inoculate three 500 mL flasks of 100 mL LB using the below volumes of this starter culture. Incubate all three flasks overnight at room temperature (18-22°C) with moderate shakingovernight. The liquid cell culture should be shaken vigorously to provide a sufficient amount of oxygen to the dividing cells. More vigorous shaking is better. . Autoclave LB agar on wet cycle for 30 min. Once the autoclave cycle is complete, check the solution to ensure that the agar is all dissolved. Alternatively if nodarken during the autoclave process if your sample has spent at least 10 min at 121 ℃. Use lab tape to label the bottle with your initials, the date, and the bottle . Autoclave, LB media, Agar plates Module Hours: 3.5 Effective Date: 03/11/2024 PRQs: Buffers and Stock Solutions . SKILLS CENTER STANDARD OPERATING
•overnight culture of bacterial colony of interest (at least 1 ml) . 1.Mix 100 % glycerol solution 1:1 with LB-Medium and autoclave the new 50 % (v/v) glycerol/LB-Medium solution to make sure it is sterile 2.Use Bunsen-burner and 70 % ethanol solution to set up sterile working area (too prevent contamination of glycerol stock, let ethanol fullyThe following protocol is for inoculating an overnight culture of liquid LB with bacteria. Video. Watch the protocol video below to learn how to inoculate bacteria in liquid culture. Protocol. . Autoclave and allow to cool to room temperature. Now screw on the top of the bottle and store the LB at room temperature.Autoclave for 20 min at 15 psi (1.05 kg/cm 2). Cool to ∼60°C and add carbenicillin (final concentration 100 µg/mL). Cool to ∼60°C and add carbenicillin (final concentration 100 µg/mL). Pour the medium into Petri dishes (∼25 mL per 100-mm plate).
NOTE: DO NOT leave anything in the autoclave OVERNIGHT!!! 7. When the cycle ends, this will be indicated on the screen by the message “Cycle Ended” (Fig #17). Follow the procedure in Step 4 to open the door, retrieve the items, and bring them back to the lab. NOTE: Be sure to close the autoclave door completely before leaving (Fig #18).
Why do you want to microwave it instead of autoclaving? It takes < 1 hour to make up and autoclave a supply of LB that will last 3-6 months. Most of that time is hands-off, just waiting for the autoclave cycle to complete. I actually think it would be significantly more annoying to rely on microwaving LB, since you'd have to boil it batch by batch.
The Overnight Express Autoinduction System: . (LB) broth, Terrific Broth 3 (TB) or animal-free medium (Veggie medium; EMD Biosciences), . and either autoclave to sterilize or microwave for a .
Another important lesson is that autoclave tape will start changing colors at temps below sterilization temperature, and will change to fully black well before actual sterilization occurs. It's really more of an indicator of "someone put this in an .
While preparing the LB liquid medium from LB agar, I am facing two problem (not at the same time) 1. Either my medium solidifies at room temperature. 2. or I am getting precipitates in my LB medium. I have prepared it by dissolving 32g of . The autoclave tape will darken during the autoclave process if your sample has spent at least 10 min at 121 ℃. Use lab tape to label the bottle with your initials, the date, and the bottle contents. This will clear up any confusion later if your forget your bottle in the autoclave. Autoclave the LB Broth and LB Agar for 15min at 121qC. Turn the autoclave off. Wait for the autoclave to cool and the pressure to become zero. . Inoculate the remaining LB broth with 5ml overnight culture that will be supplied by your teacher. Gently swirl the flask in a circular motion to thoroughly mix. 3. Transfer 1ml of your culture to a . a fresh piece of autoclave tape on the top. • Autoclave (121°C, 20 minutes) on LIQUID cycle, be sure to add water to the autoclave basin before starting the cycle! (this creates the necessary vapor pressure to prevent the liquid from evaporating in the autoclave) • Cool to RT before use. Do not tighten cap until cool.
Adhering to proper procedures, understanding compatible and incompatible materials, and ensuring regular autoclave validation are essential for the successful and safe use of autoclaves. With the knowledge gained from this guide, you are now equipped to utilize autoclaves effectively and contribute to the maintenance of a clean and sterile laboratory .
Autoclave on liquid setting (autoclave program #7) Day 1: Make LB (place flasks in 37C room overnight for faster growing tomorrow) Start overnight culture: 30 mL LB in sterile flask + 30 uL of antibiotic(s), inoculate with glycerol stock Shake at 37C overnight (12-16 hours) Day 2: For each 1 L flask of LB, add:F) but cannot measure th e length of time spent at 121. o. C (250. o. F). Autoclave User Safety Precautions: Because an autoclave uses saturated steam under high pressure to achieve sterilizing temperatures, proper use is important to ensure operator safety. Prevent injuries when using the autoclave by observing the following rules: 1. Mix 30.5 g in 1L water, heat to dissolve; autoclave and cool before use: Overnight Express Instant LB Medium: Overnight Express Autoinduction System 1 components; Luria Bertani (LB) broth; 71757-M: Granulated medium: Just add the EasyPak contents to 1 L sterile water, supplement with 10 mL glycerol, and microwave for 2 min to dissolve.
We closed the lid halfway and wrapped it with foil, pasted autoclave tape on it. 5. We stored it at 4°C since there was a problem in the autoclave. . One colony from the petri dish was taken into 5 ml of liquid LB medium and incubated overnight at 37°C at 160 rpm (to increase yield). 6. The overnight culture was collected by centrifugation .Le bouillon LB , également connu sous le nom de milieu LB, bouillon de lysogénie, bouillon Luria ou milieu Luria-Bertani, est un milieu riche en nutriments couramment utilisé pour la culture de bactéries.. Le milieu LB convient à la culture non sélective de souches d'E. coli pour le clonage, la production de plasmide d'ADN et la production de protéines recombinantes.
Inoculate 5ml LB containing the appropriate antibiotic with a single colony. Shake overnight at 37oC. 3. Inoculate 500 ml LB containing the appropriate antibiotic with the 0.5ml of the overnight. . 1. Pellet cells in SLA1500 rotor at 6000 rpm for 10min. Pour off the spent medium. 2. Quick spin cells down to pull down the last bit of medium .

Autoclave machines are an efficient and dependable way to sterilize tattoo and nail salon equipment. Autoclaves reduce the danger of infection transfer by submitting reusable tools and equipment to high-pressure saturated steam.
lb spent in the autoclave overnight|how long does lb last after autoclave